Change of atomic radius
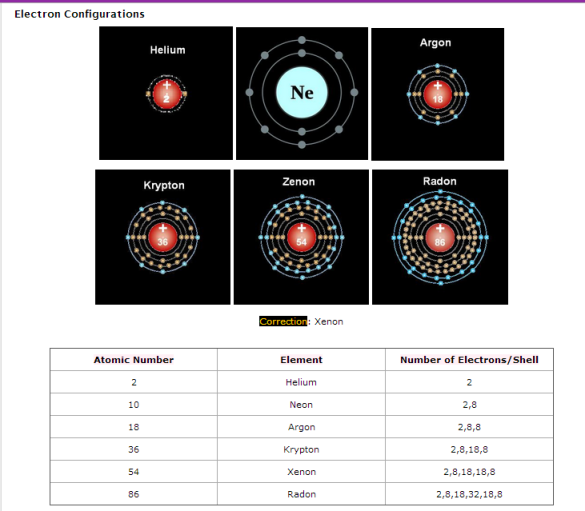
In alkali metals, the atomic radius increases down the group. Each element has 1 electron in each outer shell because they are all in group 1.
Reactivity
The alkali metals are very reactive, but they are not found in elemental forms in nature. The elements are usually found in mineral oil, or paraffin oil. Alkali metals are mostly soft silver-colored, of low density.
Uses of Alkali Metals
Lithium is used to produce ceramics and glasses.
Devices that require batteries, for example mobiles or computers contain Lithium batteries.
Sodium is used to produce salt , in the form of sodium chloride.
Sodium hydroxide is used to clean ovens.
Cesium is use to produce military aircrafts.
A compound of rubidium, silver and iodine, is used to make film batteries.
Francium doesn’t have many uses, but it’s used in laboratories for experiments, and needs to be handled carefully, because it’s toxic.
Location on the periodic table
Elements in the same group have similar, but not identical characteristics. This is because they all have the same number of electrons in their outer shell. You can tell a lot about an element, if you know which group it belongs to.
Abundance on earth
All of the alkali metals are found in nature, but not in their pure forms. Most combine with oxygen and silica to form minerals in Earth, and have to get separated from the substance that they’re combined with. Alkali metals are soft silver-colored, with low density and can explode when combined with water.
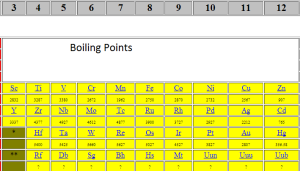
Change of boiling point and melting point
All alkali metals are very soft and they have low melting and boiling points. Alkali metals melting and boiling points decrease down the group, so which means that Lithium has a lower boiling and melting point than Francium. As you go down, the alkali metals, the melting and boiling points get lower.

Note: Hydrogen is not an Alkali metal, but hydrogen is in group 1 because it only has 1 electron shell on the outer electron shell.
Hydrogen (H)
Hydrogen doesn’t belong to a group on its own, because it has its own differences and needs to share the same form to fit in a group. Sometimes hydrogen is considered to be in the first group, or group 1.
Atomic Radius
Hydrogen has 1 shell, 1 proton and no neutrons, and since it doesn’t belong to a group, the atomic radius doesn’t change in its group; although it’s considered to be in the first group, with the alkali metals, because it has one shell.
Reactivity
Hydrogen is highly reactive in high temperatures with one electron and it tries to respond with other elements, to make compounds, like water. Hydrogen is a flammable substance and a colorless gas.
Uses of Hydrogen
Hydrogen can be used as a fuel for rockets.
Hydrogen and oxygen combine to make water, which is one of the most important compounds to the existent of the human race.
Location on the periodic table
Hydrogen is the first element on the periodic table, because it has 1 atom; that’s how elements have been arranged in the periodic table.
Is Hydrogen natural or man-made?
Hydrogen is natural, and can be found as a compound, and it needs to be separated to get pure hydrogen, by it self.
Change of boiling point and melting point
Melting point: -259.2°C
Boiling point: -252.762°C